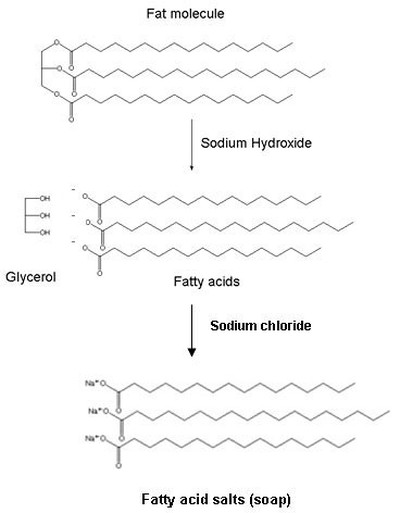
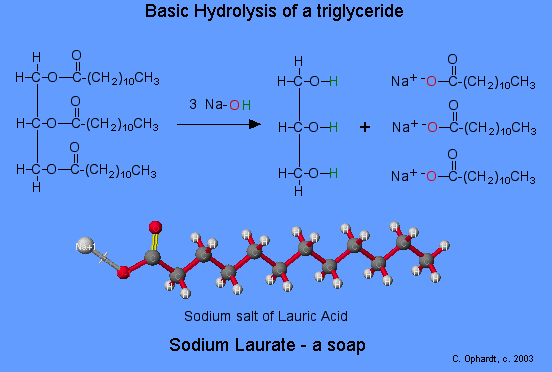
biocomiche.it - la scienza leggera da leggere: Soap or the solubility of ions, sugars and fat in water
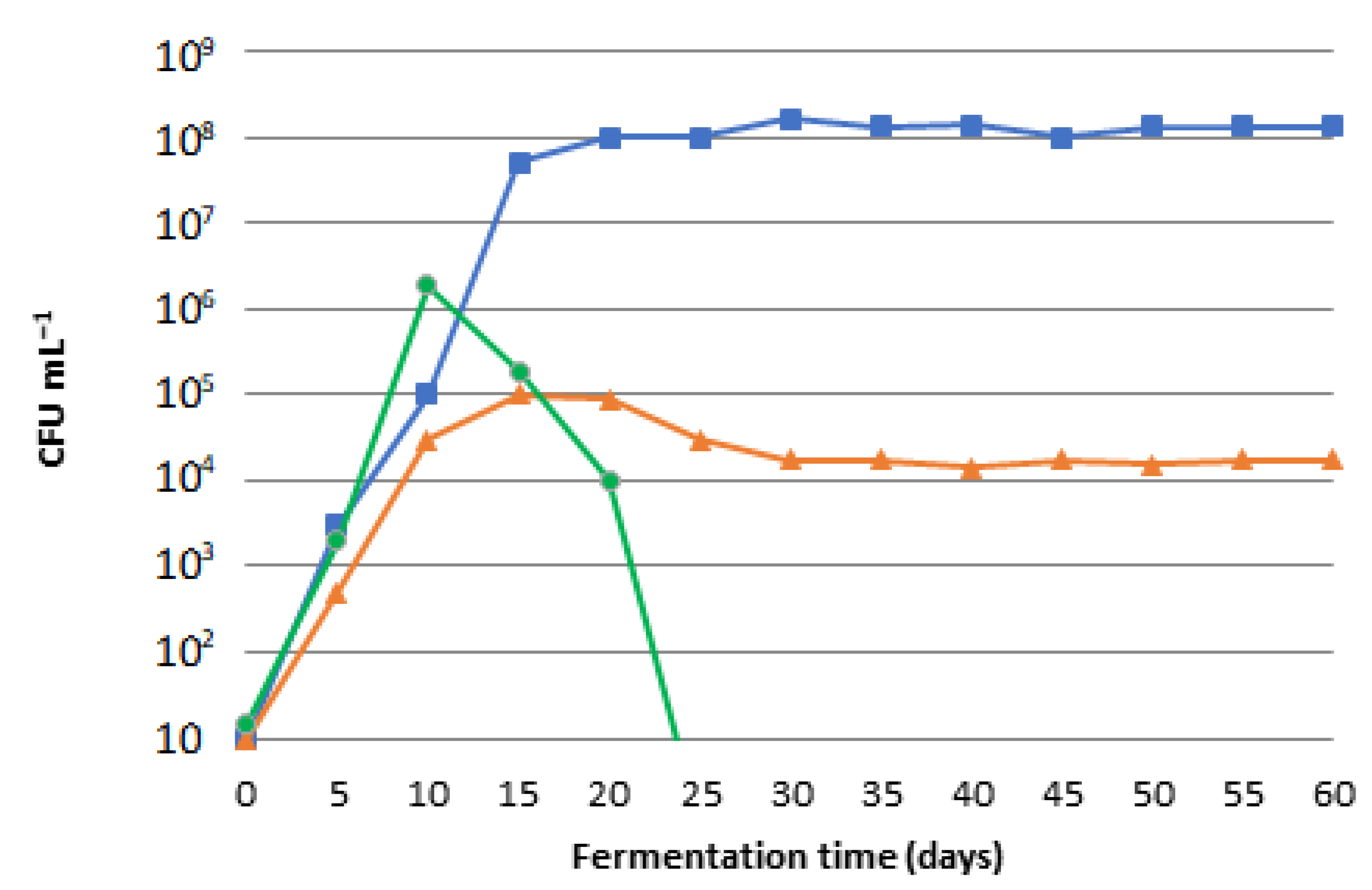
Fermentation | Free Full-Text | Production and Maturation of Soaps with Non-Edible Fermented Olive Oil and Comparison with Classic Olive Oil Soaps

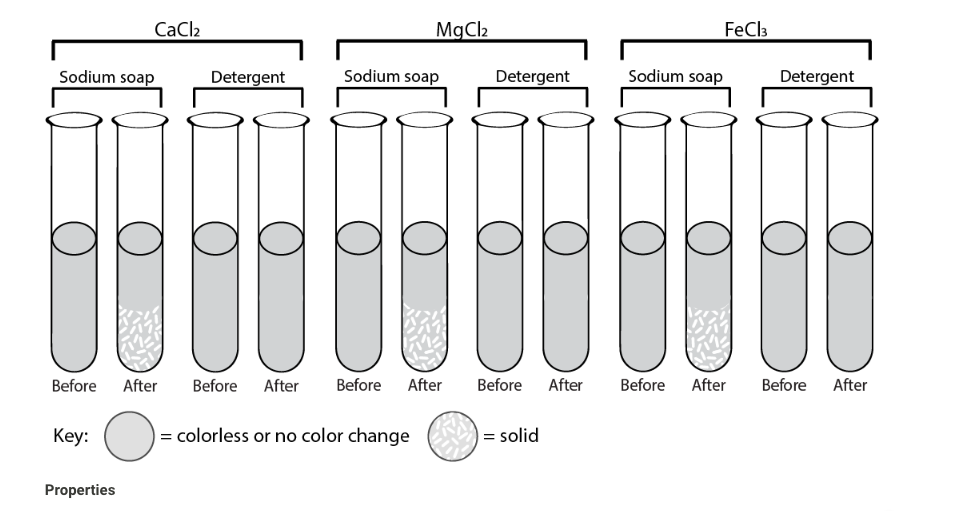
SOLVED: Question 5 Which of the following is not objective of preparation of soap experiment? a.To study the solubility of soap b.To realized that soap does clean in hard water C To

A solution containing a mixture of 0.05 M NaCI and 0.05 M Nal is taken. ( Ksp of AgCl = 10^-10 and Ksp of AgI = 4 × 10^-16 ). When AgNO3 is added to such a solution