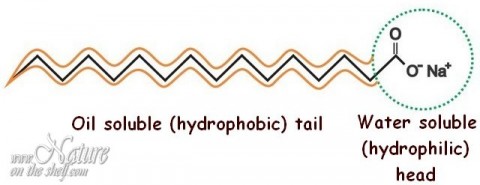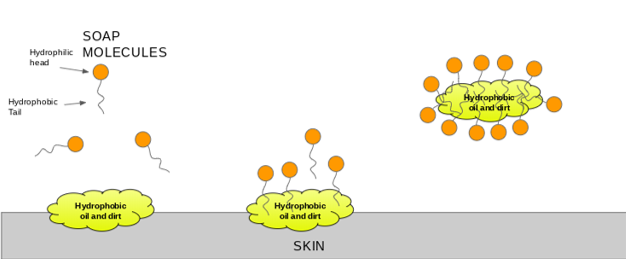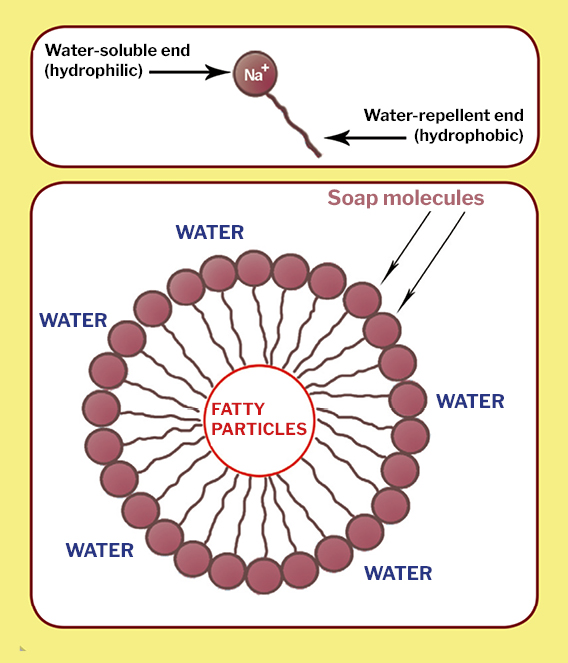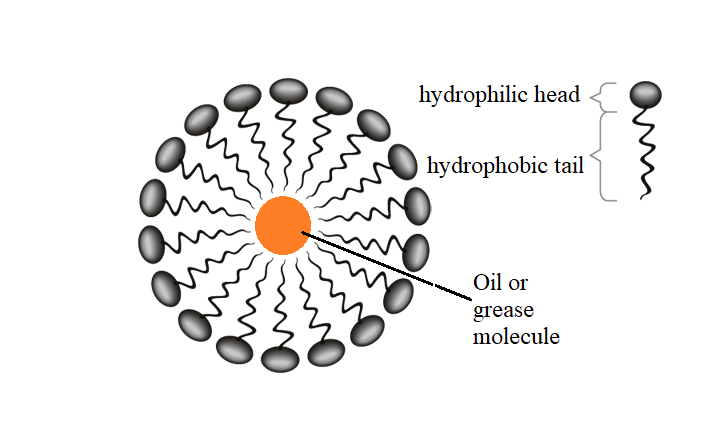
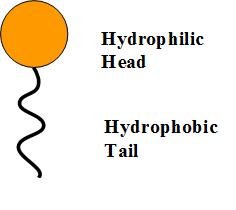
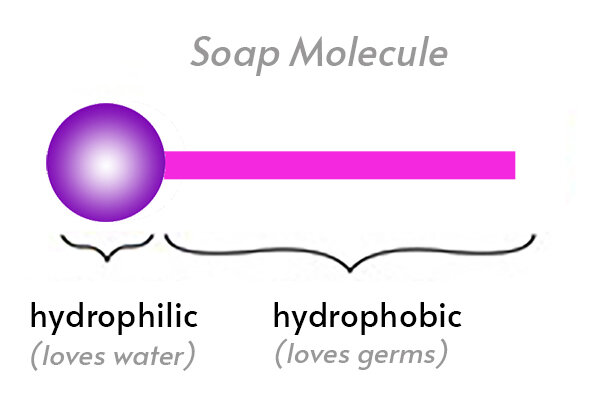
The soap molecule has a: A) Hydrophilic head and a hydrophobic tail B) Hydrophobic head and a hydrophilic tail C) Hydrophobic head and a hydrophobic tail D) Hydrophilic head and a hydrophilic
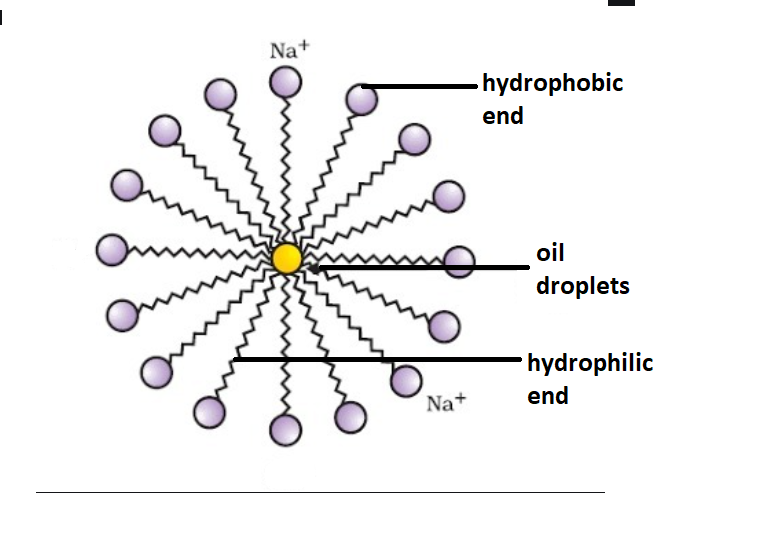
Which of the given statements is true?a.) The ionic end of soap dissolves in water while the carbon chain dissolves in oilb.) The ionic end of soap dissolves in oil while the

everyday chemistry - How does soap help in cleaning clothes and rinsing off oil and grease? - Chemistry Stack Exchange
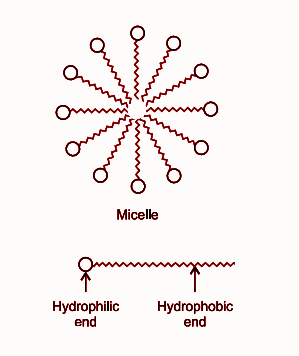
a) What is soap? (b) Describe the structure of a soap molecule with the help of a diagram. (c) Explain the cleansing action of soap. Draw a diagram to illustrate your answer.
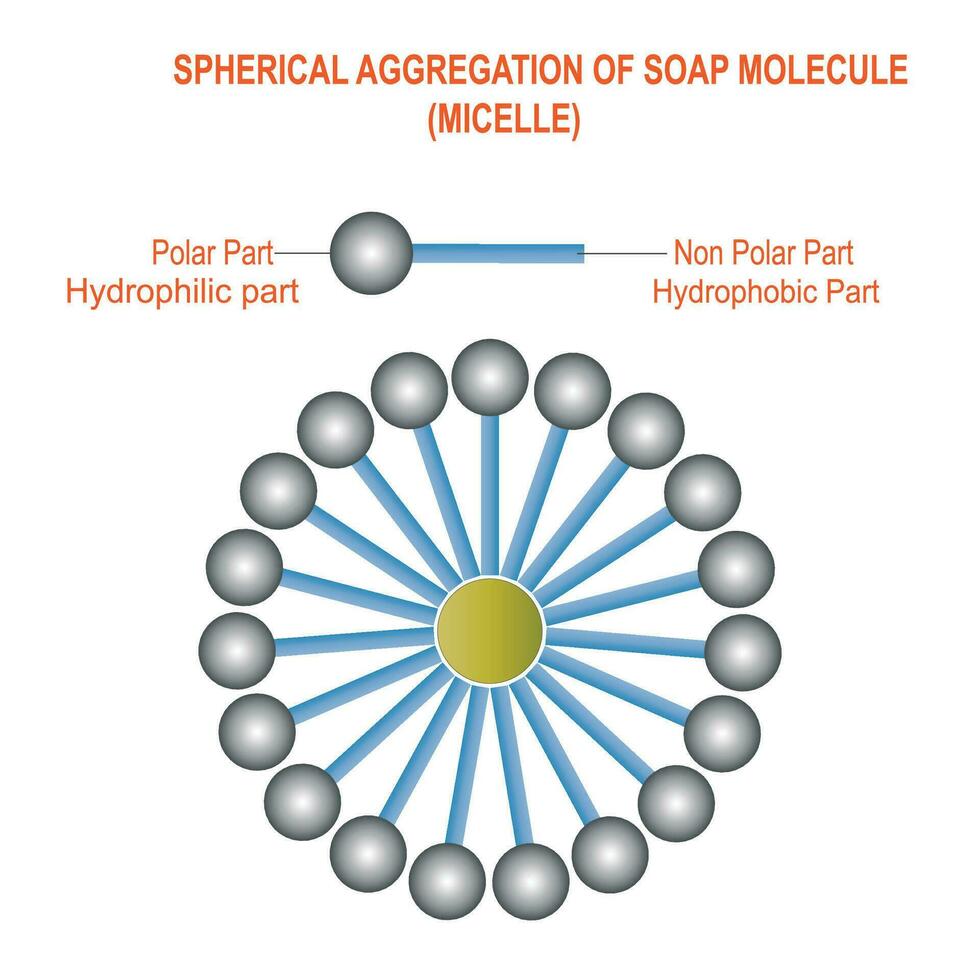



:max_bytes(150000):strip_icc()/soap-micelle-58ed36193df78cd3fcdf0908.jpg)

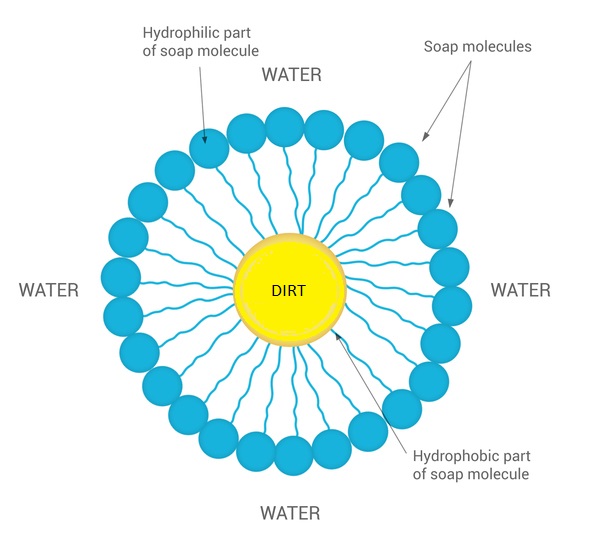



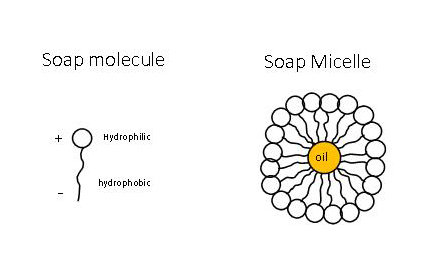
![MCQ] In the soap micelles (a) the ionic end of soap is on the surface MCQ] In the soap micelles (a) the ionic end of soap is on the surface](https://d1avenlh0i1xmr.cloudfront.net/aed2074f-aafe-447a-b1f0-adb685bf01d5/structure-of-a-soap-micelle---teachoo.jpg)