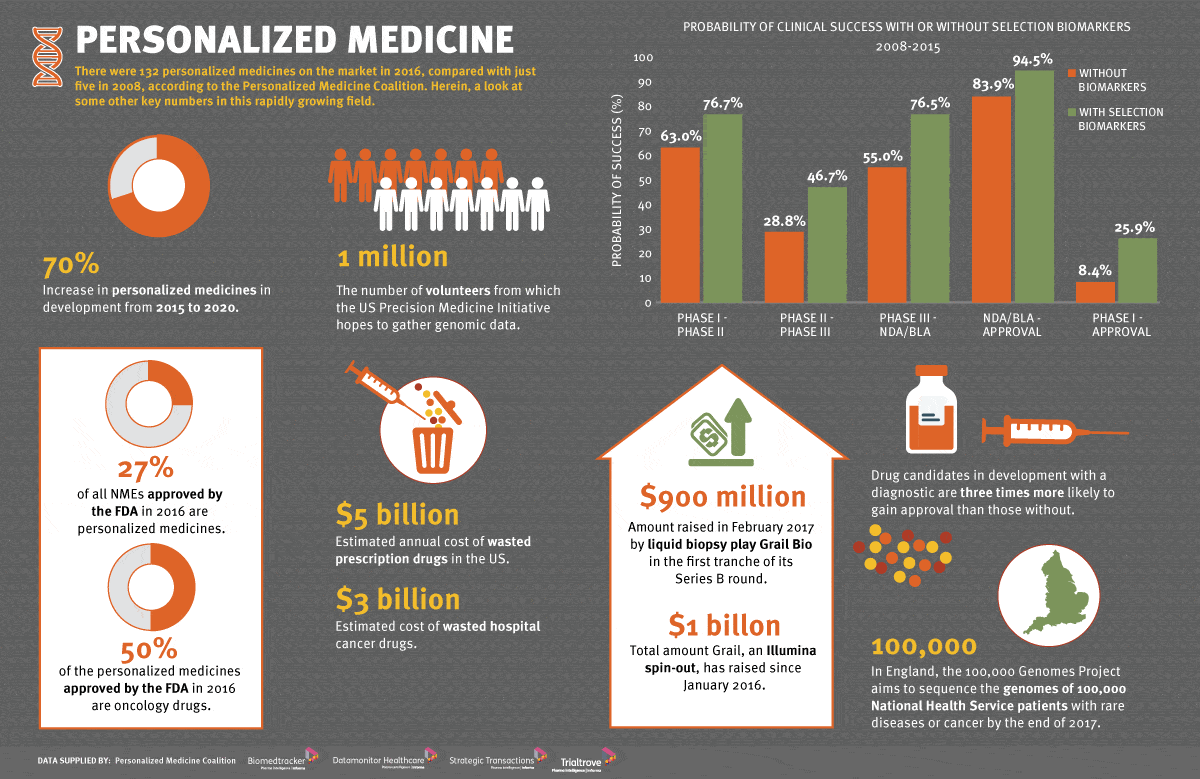
![Workflow for TMB assessment using the FoundationOne CDx assay [63].... | Download Scientific Diagram Workflow for TMB assessment using the FoundationOne CDx assay [63].... | Download Scientific Diagram](https://www.researchgate.net/publication/334081089/figure/fig2/AS:774610417090560@1561692923137/Workflow-for-TMB-assessment-using-the-FoundationOne-CDx-assay-63-ExAC-Exome.png)
Workflow for TMB assessment using the FoundationOne CDx assay [63].... | Download Scientific Diagram
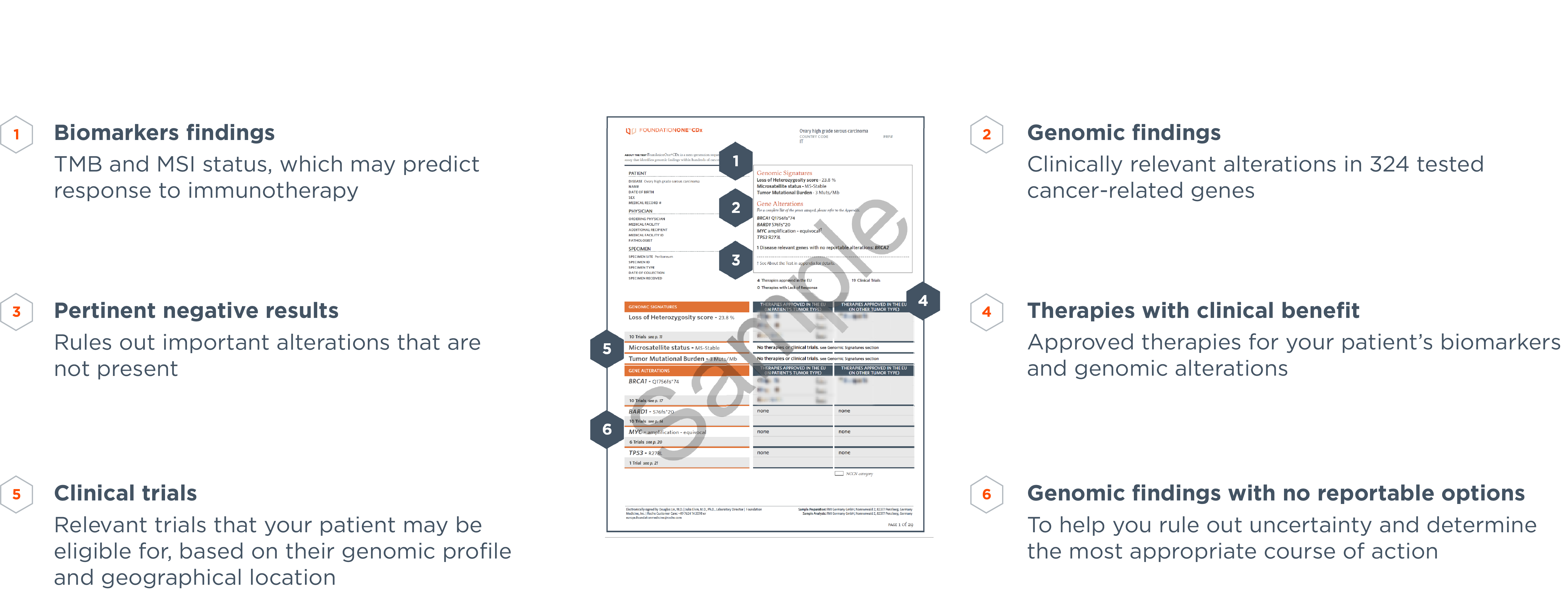
FoundationOne®CDx Technical Information Foundation Medicine, Inc. 150 Second Street, Cambridge, MA 02141 Phone: 617.418.2200 I

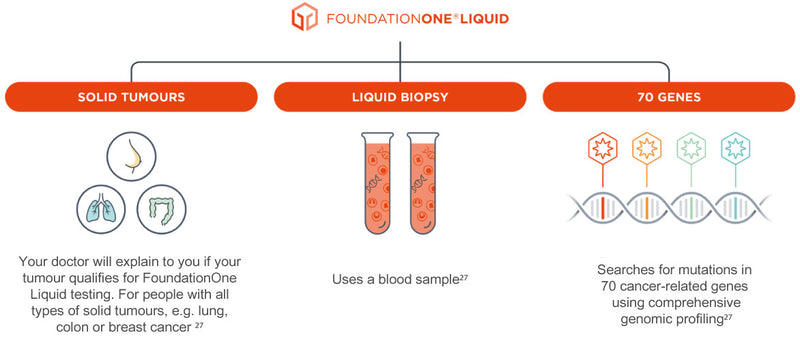
Roche Ireland on X: "FoundationOne®CDx uses a sample of your cancer tissue and FoundationOne®Liquid CDx a blood sample to provide a more complete picture of your cancer and help open up treatment

Foundation Medicine on X: "Now FDA approved as a companion diagnostic for PIQRAY® (alpelisib) to identify PIK3CA mutations, FoundationOne®CDx* may help match even more patients to approved treatments. Learn more: https://t.co/8lbnBl0RPX *Rx

Foundation Medicine and Pfizer Announce Broad Partnership to Develop Companion Diagnostics for Pfizer's Oncology Portfolio | Business Wire




![Foundation Medicine review - 7 facts you should know [DECEMBER 2021] Foundation Medicine review - 7 facts you should know [DECEMBER 2021]](https://nebula.org/blog/wp-content/uploads/2021/12/FoundationOne-Heme-testing-kit.png)